Not known Factual Statements About Antigen Testing Recommendations – Coronavirus
 BD secures order for Covid-19 antigen tests from Canada
BD secures order for Covid-19 antigen tests from CanadaSome Known Incorrect Statements About Ellume Covid-19 Rapid Antigen Home Test - Target

Will this test tell me if I have been infected with COVID-19 in the past? No, this is an antigen test that just finds an active infection. Antibody tests can discover past infections. Can I use this COVID test to fulfill travel requirements for testing? No, this is for personal use only and doesn't supply a recorded test result that you can display when taking a trip.
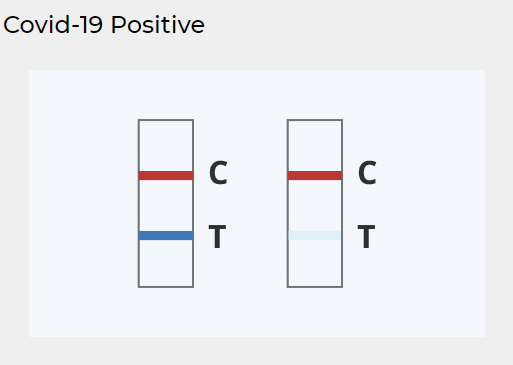 COVID-19 Rapid Antigen Tests - CareStart™ - RCHM-02071 - Code 1 Supply
COVID-19 Rapid Antigen Tests - CareStart™ - RCHM-02071 - Code 1 SupplyThis test is used by e, Med, please check out for further info. Does the product expire? For how long is it great for? Each item box has an expiration date and must not be utilized after that date. Do I need any additional products to conduct the test in your home? Yes, you will require a timer/watch and it is suggested that gloves are worn during testing.
By evaluating more frequently, you may find COVID-19 quicker and decrease spread of infection. What do I require to understand about Results from Serial Testing? If your first test is negative, you should test again within 3 days, with a minimum of 36 hours between tests. If your very first or second test is favorable, then proteins from the virus that causes COVID-19 have been found in your specimen and you likely have COVID-19.
 Comparison of seven commercial SARS-CoV-2 rapid point-of-care antigen tests: a single-centre laboratory evaluation study - The Lancet Microbe
Comparison of seven commercial SARS-CoV-2 rapid point-of-care antigen tests: a single-centre laboratory evaluation study - The Lancet MicrobeYou may require additional testing, depending on your individual health history and other factors. What is Emergency Situation Usage Authorization (EUA)? FDA emergency gain access to mechanism. Health & Human Services declare when circumstances exist to justify usage of diagnostics under EUA throughout public health emergency situations. I Found This Interesting is not full FDA clearance or approval and is temporary, until the statement is terminated or withdrawed.
An Unbiased View of Rapid Antigen/Near-Patient/PoC Tests for COVID-19
We have performed a comprehensive analysis of known variants that we have actually had the ability to study, and we are confident that our tests remain effective at identifying these stress. It is extremely likely that future COVID-19 pressures will remain detectable due to the fact that our tests and most other COVID-19 tests look for parts of the infection that are vital for the infection' survival and therefore less based on anomaly.
