Getting My FDA adds another COVID-19 test to 'do not use' list To Work
The Single Strategy To Use For FlowFlex COVID 19 Antigen Rapid Test, 1 ct - Fred Meyer
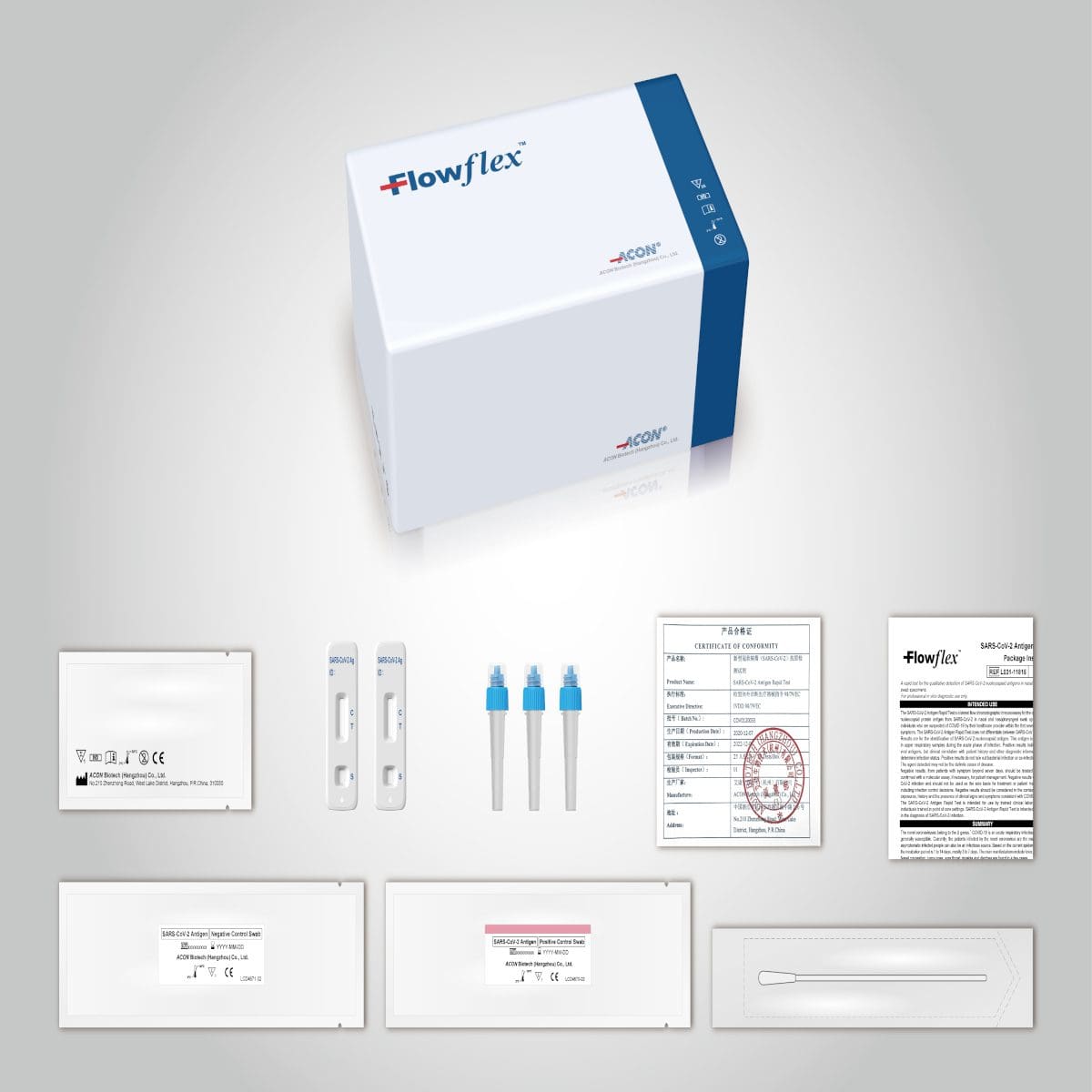
The Flowflex COVID-19 Antigen House Test is an easy, affordable way to figure out COVID-19 status whether symptoms are present or not. It just takes 4 steps and 15 minutes to finish the test. The test is likewise non-invasive: You will not need to gather a sample from deep in your nose cavity to get accurate results.
 FlowFlex OTC Rapid Antigen Test (288 Tests) ($10 per test) - CrowdHealth
FlowFlex OTC Rapid Antigen Test (288 Tests) ($10 per test) - CrowdHealthA fast test for the detection of SARS-Co, V-2 antigens in anterior nasal specimens straight from people within 7 days of symptom beginning or without signs or other epidemiological factors to presume COVID-19 infection. Qualitative detection of the nucleocapsid protein antigen from SARS-Co, V-2 in anterior nasal swab specimens directly from individuals within 7 days of sign beginning or without signs or other epidemiological reasons to think COVID-19 infection.
The Flowflex COVID-19 Antigen Home Test does not need serial screening. "In the U.S.A., this item has not been FDA cleared or authorized, but has been licensed by FDA under an EUA. Check Here For More has been licensed only for the detection of proteins from SARS- Co, V-2, not for any other viruses or pathogens.
360bbb-3(b)( 1 ), unless the declaration is ended, or permission is withdrawed quicker." * FREE GROUND shipping applies to 48 Continental States. Extra shipping fee requests areas outside continental U.S.A..
 Flowflex SARS-CoV-2 Antigen Rapid Test Video (English) - YouTube
Flowflex SARS-CoV-2 Antigen Rapid Test Video (English) - YouTubeThe Main Principles Of FDA adds another COVID-19 test to 'do not use' list - WKYC

The U.S. Fda has issued cautions about three more at-home COVID-19 test packages that it has not licensed and may give false outcomes. The makers of all 3 tests also have actually provided recalls. The FDA issued warnings about these tests the other day: The FDA stated consumers need to not use the Celltrion Dia, Trust COVID-19 Ag Rapid Test that has been remembered.
 US FDA warns against use of unauthorised Covid-19 antigen tests including SD Biosensor, Flowflex kits - TODAY
US FDA warns against use of unauthorised Covid-19 antigen tests including SD Biosensor, Flowflex kits - TODAYThe FDA is worried about the threat of incorrect results when utilizing this unapproved test."The FDA stated ACON Laboratories has recalled all the Flowflex tests. SD Biosensor has actually recalled its tests and Celltrion USA has remembered the Dia, Trust tests.In February, the FDA had actually released warnings about the E25Bio COVID-19 Direct Antigen Quick Test, the Empowered Diagnostics Cov, Clear COVID-19 Quick Antigen Test and Immuno, Pass COVID-19 Neutralizing Antibody Rapid Test for the exact same factors.
